
Dynamic evolution and reversibility of single-atom Ni(II) active site in 1T-MoS2 electrocatalysts for hydrogen evolution | Nature Communications

SOLVED: Select the correct balanced net ionic equation for each of the following reactions occuring in aquequssolution Cu(NO3h2 Ni(NO3)2 AgNO3 NaCl Agcl NaNO3 Ca(NO3h2 KzCo3 Caco3 2KNO3 Cuz+ 2NO3 Ni(NO3)2 Ag+ NaNO3

Direct transformation of AgNO3 complex encapsulated Fullerene (C60) microcrystal on solid silver Nitrate Crystal without organic Ligands - Hou - 2020 - Applied Organometallic Chemistry - Wiley Online Library

SOLVED: 25.Which of the following would you expect to produce a reaction When mixed together? Ag(s) + Co(NOa)z(aq) Pb(s) Co(NOs)z(aq) Sn(s) + Ni(NOs)2(aq) Ni(s) + AgNOs(aq) Pb(NOs)zlaq) Co(NO3) (aq)
1 REVIEW Unit 4 Test (Chp 4): Aqueous Reactions and Solution Stoichiometry Multiple Choice polyatomic ions (names, charges, form

Depth Ni profile analyses of XPS. The profile indicates that after Ni... | Download Scientific Diagram

Overcoming Limitations in Decarboxylative Arylation via Ag–Ni Electrocatalysis | Journal of the American Chemical Society
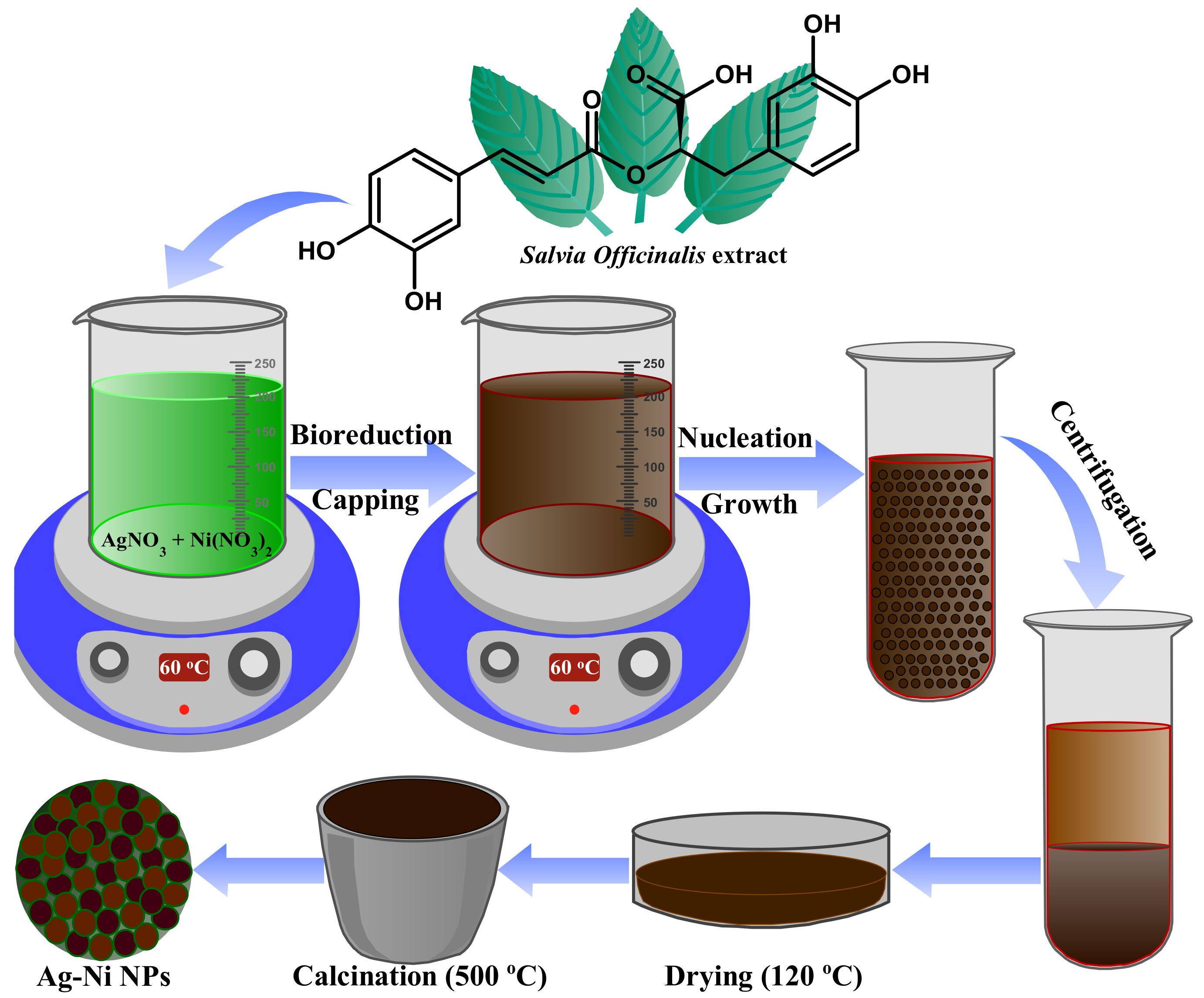
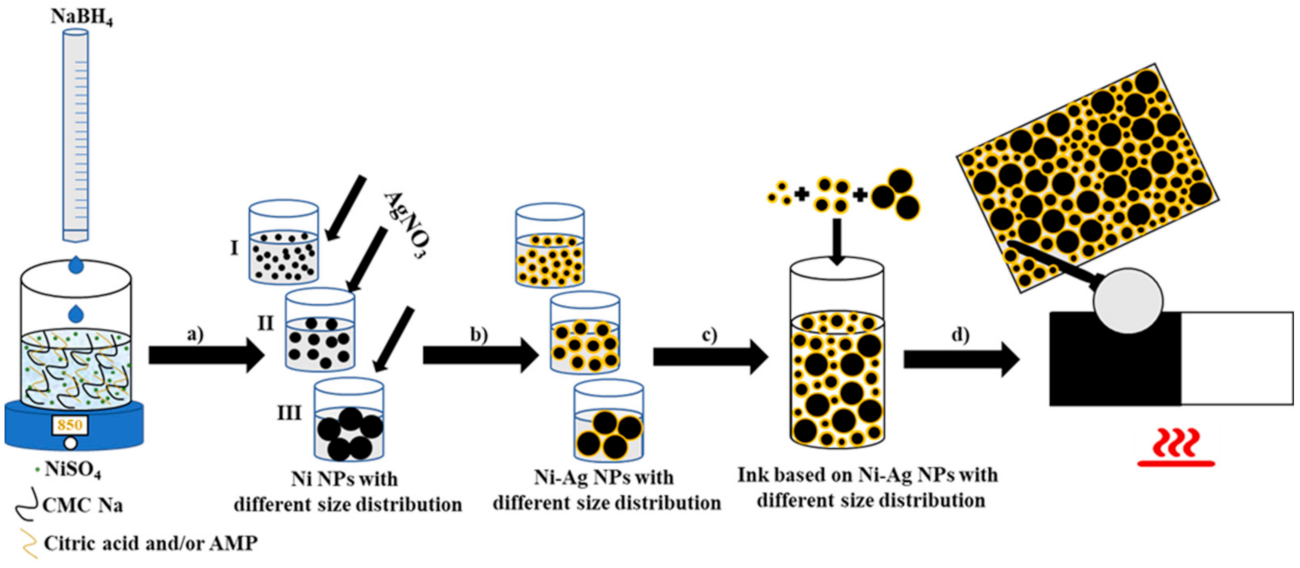
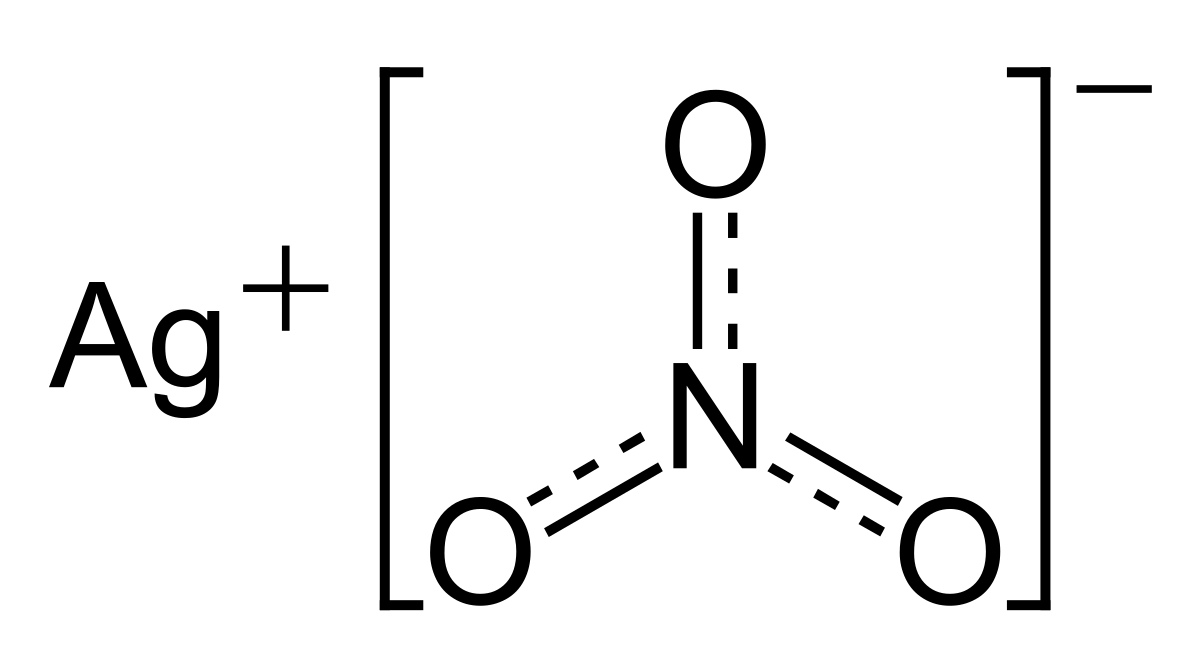
JoF | Free Full-Text | Combination Effect of Novel Bimetallic Ag-Ni Nanoparticles with Fluconazole against Candida albicans

OneClass: 9. Based on the equations below, which metal is the most active? Pb(NO3)2 (aq) + Ni (s) â†'...

Three faraday of electricity is passed through molten solutions of AgNO3, NiSO4 and CrCl3 kept in three vessels using inert electrodes. The ratio in mol in which the metals Ag, Ni and







![The complex of [Co(NH3)5Br]Cl , the ionization isomer will give colour with AgNO3 . The complex of [Co(NH3)5Br]Cl , the ionization isomer will give colour with AgNO3 .](https://toppr-doubts-media.s3.amazonaws.com/images/7410843/66afa896-6332-4a2e-a0c9-acc6154c6ca9.jpg)
