Explain [Co(NH_3)_6]^{3+} is an inner orbital complex whereas [Ni(NH_3)_6]^{2+} is an outer orbital complex.

Table 4 from Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate | Semantic Scholar
![Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:598/0*gC7TqoG4Szy6QwnB.jpg)
Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
![C) 10 5. Which one of the following is an outer orbital complex and exhibits paramagnetin behaviour? (a) [Ni(NH3)6]2+ (b) [Zn(NH3)612+ (c) [Cr( NH3)6]3+ (d) (CO(NH3)6]3+ - T r action A + B C) 10 5. Which one of the following is an outer orbital complex and exhibits paramagnetin behaviour? (a) [Ni(NH3)6]2+ (b) [Zn(NH3)612+ (c) [Cr( NH3)6]3+ (d) (CO(NH3)6]3+ - T r action A + B](https://toppr-doubts-media.s3.amazonaws.com/images/8783536/3c02ac26-f23b-4dda-96f6-23fb3b82533e.jpg)
C) 10 5. Which one of the following is an outer orbital complex and exhibits paramagnetin behaviour? (a) [Ni(NH3)6]2+ (b) [Zn(NH3)612+ (c) [Cr( NH3)6]3+ (d) (CO(NH3)6]3+ - T r action A + B
![Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:1280/0*8wwL8Ru43LyLIIL8.png)
Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
Coordination compound: Explain [Co(NH3)6]3+ is an inner orbital complex while [Ni(NH3)6]2+ is an outer orbital complex .
2 – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub. Thermal decomposition of polycrystalline [Ni(NH3)6](NO3)2 – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub.](https://cyberleninka.org/viewer_images/173973/f/1.png)
Thermal decomposition of polycrystalline [Ni(NH3)6](NO3)2 – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub.
![SOLVED: [Ni(OH2)]2+ is green; while [Ni(NH3)]2+ is purple. Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6]2+ absorbs violet light. [Ni(OH2)]2+ absorbs red light. SOLVED: [Ni(OH2)]2+ is green; while [Ni(NH3)]2+ is purple. Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6]2+ absorbs violet light. [Ni(OH2)]2+ absorbs red light.](https://cdn.numerade.com/ask_images/6feedc9774204ebeabd886be4a6d6f35.jpg)
SOLVED: [Ni(OH2)]2+ is green; while [Ni(NH3)]2+ is purple. Which of the following statements is incorrect? [Ni(OH2)6]2+ will absorb light of lower energy than [Ni(NH3)6]2+. [Ni(NH3)6]2+ absorbs violet light. [Ni(OH2)]2+ absorbs red light.
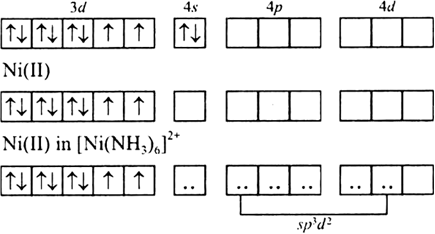
![Explain the hybridisation, magnetic property and geometry { left[ Ni{ left( CN right) }_{ 4 } right] }^{ 2- } and { left[ Ni{ left( { NH }_{ 3 } right) }_{ 4 } right] }^{ 2+ } using VB theory. Explain the hybridisation, magnetic property and geometry { left[ Ni{ left( CN right) }_{ 4 } right] }^{ 2- } and { left[ Ni{ left( { NH }_{ 3 } right) }_{ 4 } right] }^{ 2+ } using VB theory.](https://search-static.byjusweb.com/question-images/toppr_ext/questions/633645_607318_ans_06ef044c85004ad2aed957fc1fd1f24d.png)
Explain the hybridisation, magnetic property and geometry { left[ Ni{ left( CN right) }_{ 4 } right] }^{ 2- } and { left[ Ni{ left( { NH }_{ 3 } right) }_{ 4 } right] }^{ 2+ } using VB theory.

![The oxidation no. of 'Ni' in [Ni(NH3)6]SO4 complex is - Brainly.in The oxidation no. of 'Ni' in [Ni(NH3)6]SO4 complex is - Brainly.in](https://hi-static.z-dn.net/files/d38/7903db0c69d1687211066aec4221c6cc.jpg)
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-5256b41f5879418f6611bf7cb42000b7.webp)
![Telugu] Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni Telugu] Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C07_E04_030_S02.png)
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-f7fd36508df0305723aa4956637d3097.webp)
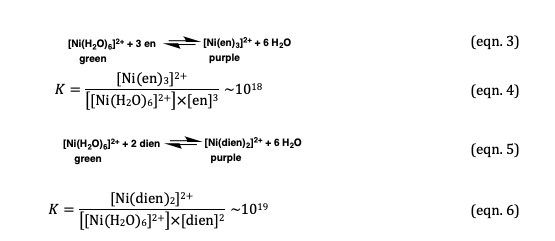


2. The... | Download Scientific Diagram Representation of the cubic structure of [Ni(NH3)6](NO3)2. The... | Download Scientific Diagram](https://www.researchgate.net/publication/269400063/figure/fig8/AS:667921089036294@1536256205990/Representation-of-the-cubic-structure-of-NiNH36NO32-The-constituent-atoms.jpg)

![Beautiful Crystals of [Ni(NH3)6]Cl2... - Chemistry is love | Facebook Beautiful Crystals of [Ni(NH3)6]Cl2... - Chemistry is love | Facebook](https://lookaside.fbsbx.com/lookaside/crawler/media/?media_id=676374042957917)


![Characteristic bands in infrared spectrum of [Ni(NH3) 6 ][VO(O 2 ) 2... | Download Table Characteristic bands in infrared spectrum of [Ni(NH3) 6 ][VO(O 2 ) 2... | Download Table](https://www.researchgate.net/publication/256461217/figure/tbl1/AS:667594596024340@1536178363556/Characteristic-bands-in-infrared-spectrum-of-NiNH3-6-VOO-2-2-NH-3-2.png)