Preparation of nickel-iron hydroxides by microorganism corrosion for efficient oxygen evolution | Nature Communications
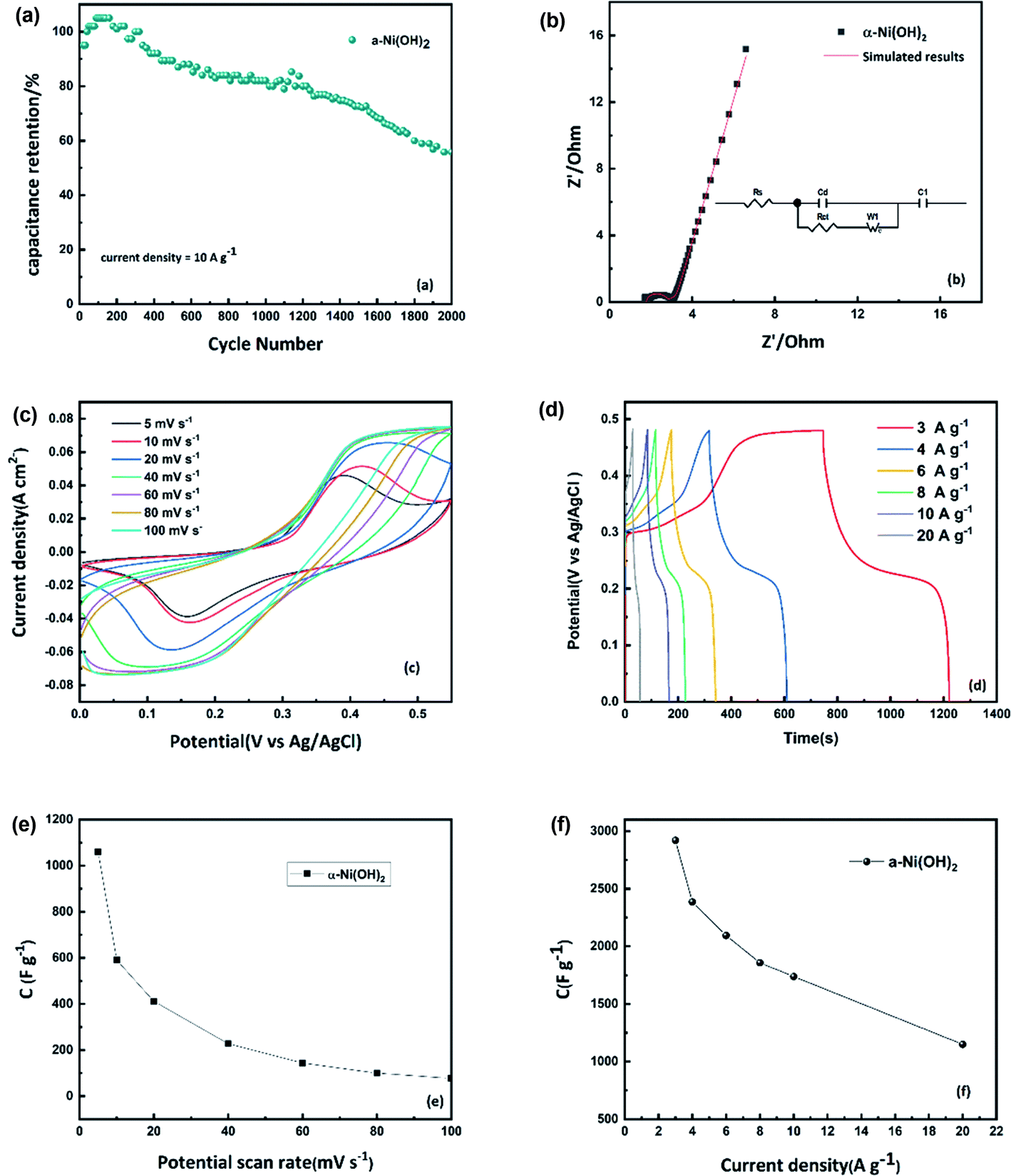
Nanoflower Ni(OH) 2 grown in situ on Ni foam for high-performance supercapacitor electrode materials - Sustainable Energy & Fuels (RSC Publishing) DOI:10.1039/D1SE01036K
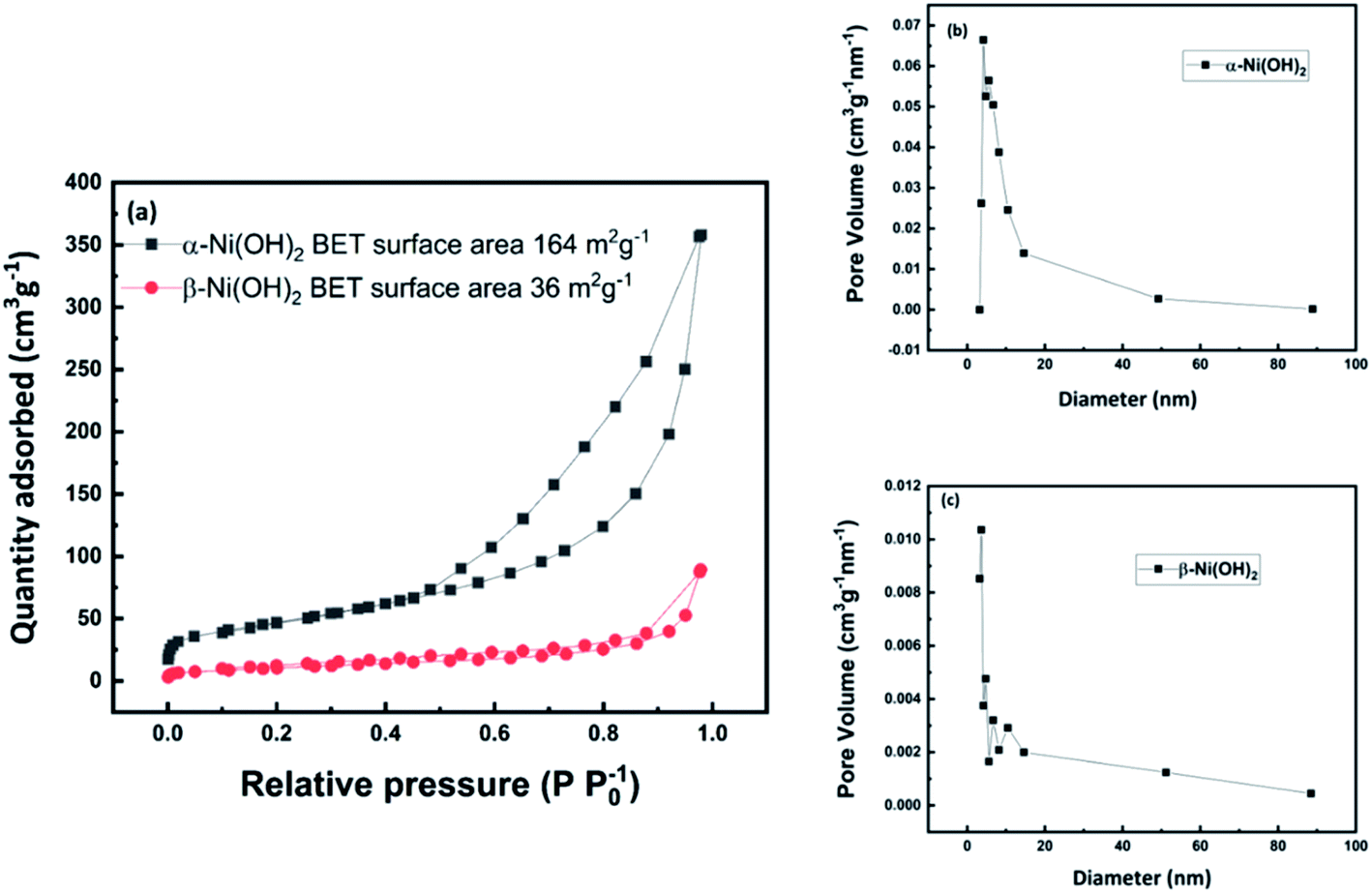
Nanoflower Ni(OH) 2 grown in situ on Ni foam for high-performance supercapacitor electrode materials - Sustainable Energy & Fuels (RSC Publishing) DOI:10.1039/D1SE01036K

Surface modulated Fe doping of β‐Ni(OH)2 nanosheets for highly promoted oxygen evolution electrocatalysis - Hu - 2022 - EcoMat - Wiley Online Library

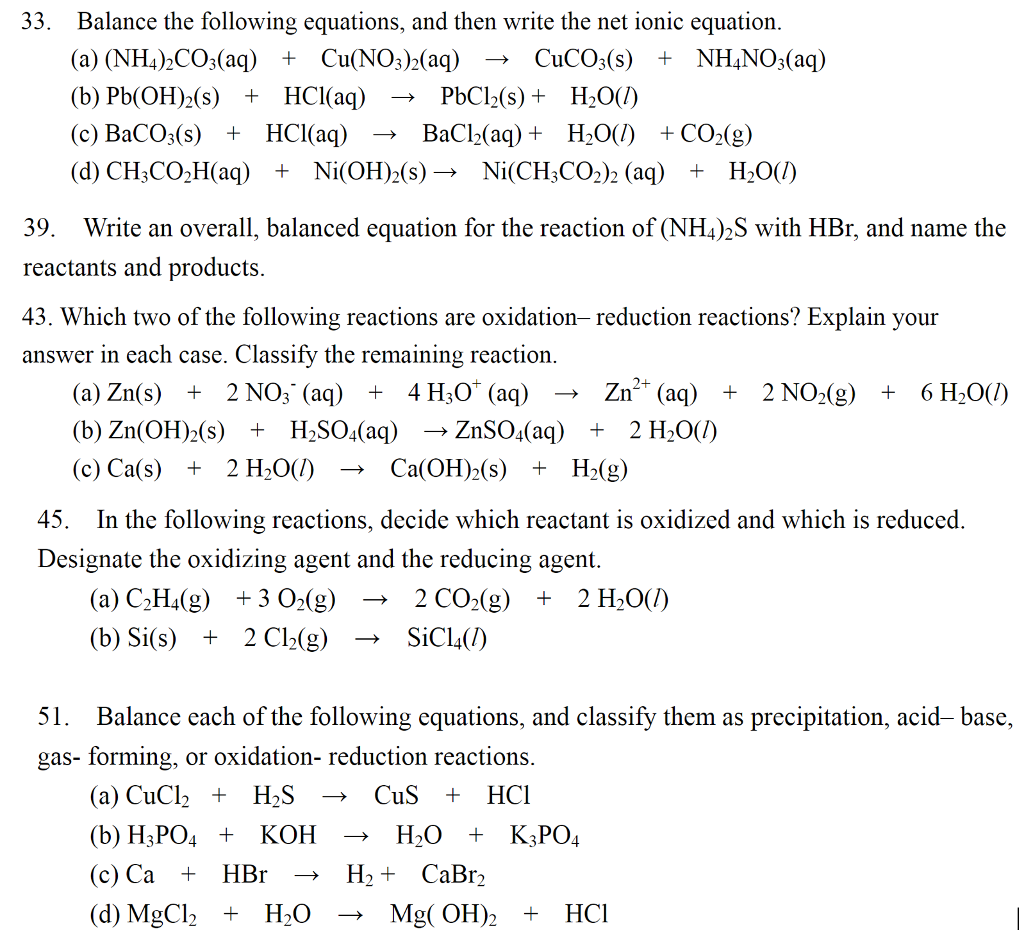
Complete and balance the following molecular equation. Al(OH)_3(s) + HNO_3(aq) to And write the net ionic equation for it. | Homework.Study.com

One material, multiple functions: graphene/Ni(OH)2 thin films applied in batteries, electrochromism and sensors | Scientific Reports

Chapter 4: Aqueous Reactions Solution: Solvent: substance present in the larger amount Solute: substance(s) dissolved in solvent, generally present in. - ppt download

Nickel hydroxides and related materials: a review of their structures, synthesis and properties | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences

Morphology-controllable nanocrystal β-Ni(OH)2/NF designed by hydrothermal etching method as high-efficiency electrocatalyst for overall water splitting - ScienceDirect
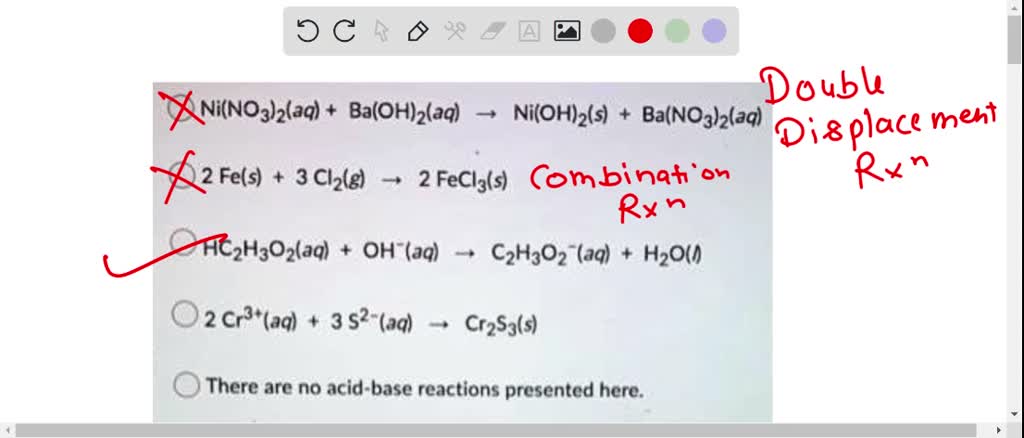
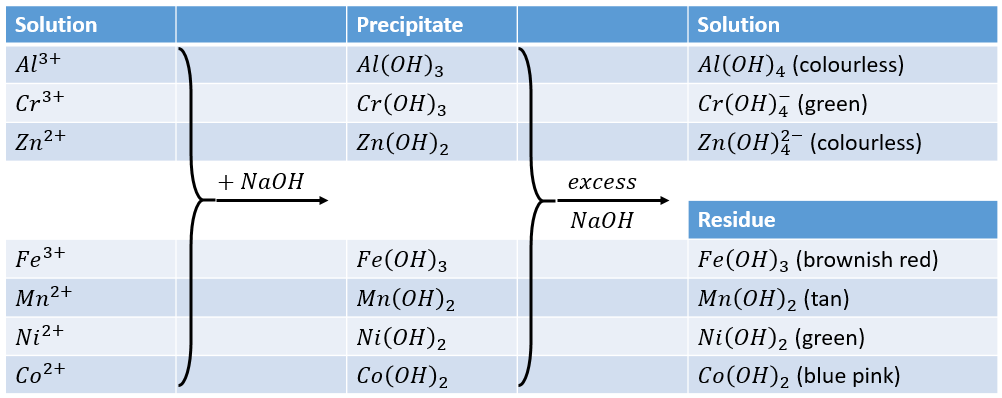
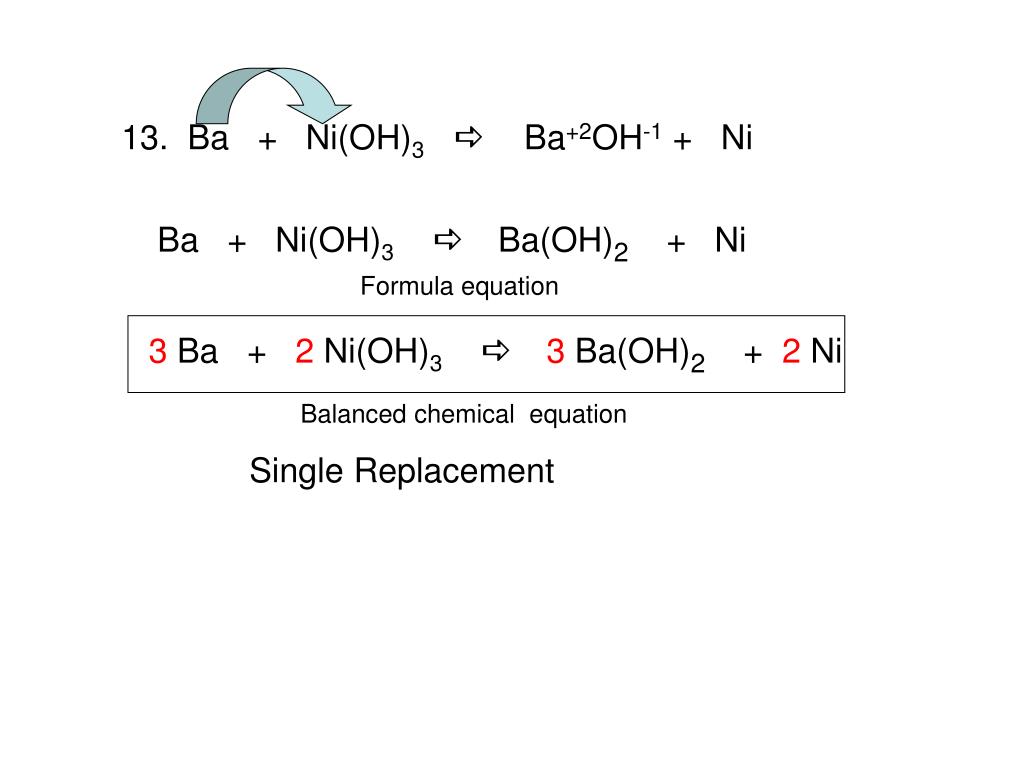
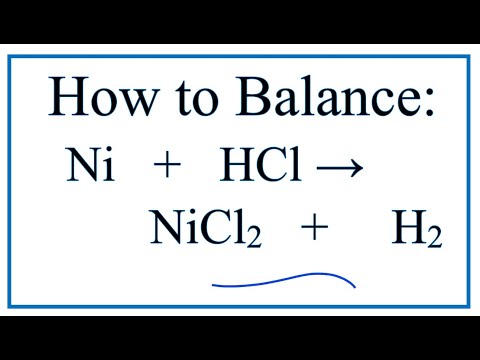
SOLVED: Question 14 (1 point) Which of the following reactions could be classified as an acid-base reaction? Ni(NO3)2(aq) + Ba(OH)2(aq) â†' Ni(OH)2(s) + Ba(NO3)2(aq) 2 Fe(s) + 3 Cl2(g) â†' 2 FeCl3(s)

Porous Fe-Doped β-Ni(OH)2 Nanopyramid Array Electrodes for Water Splitting | ACS Applied Materials & Interfaces
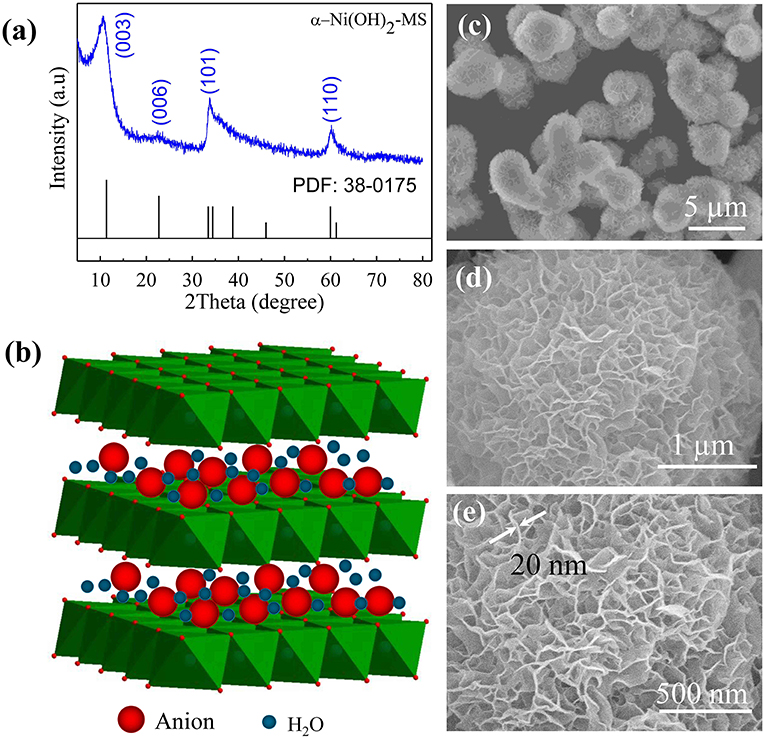
Frontiers | Facile Synthesis of Monodispersed α-Ni(OH)2 Microspheres Assembled by Ultrathin Nanosheets and Its Performance for Oxygen Evolution Reduction

Find out the solubility of Ni(OH)_2 in 0.1 MNaOH. Given that the ionic product of Ni(OH)_2 is 2 ×... - YouTube