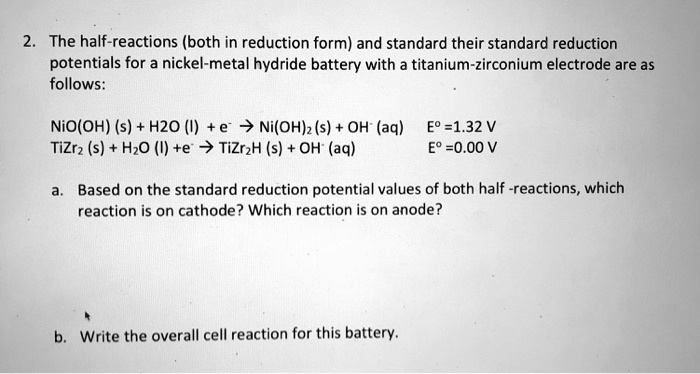
![Expert Answer] calculate standard electrode potential of Ni/Ni2+,if cell potential of the - Brainly.in Expert Answer] calculate standard electrode potential of Ni/Ni2+,if cell potential of the - Brainly.in](https://hi-static.z-dn.net/files/d51/b7359f4685f931af8cd83cf0636289d3.jpg)
Expert Answer] calculate standard electrode potential of Ni/Ni2+,if cell potential of the - Brainly.in
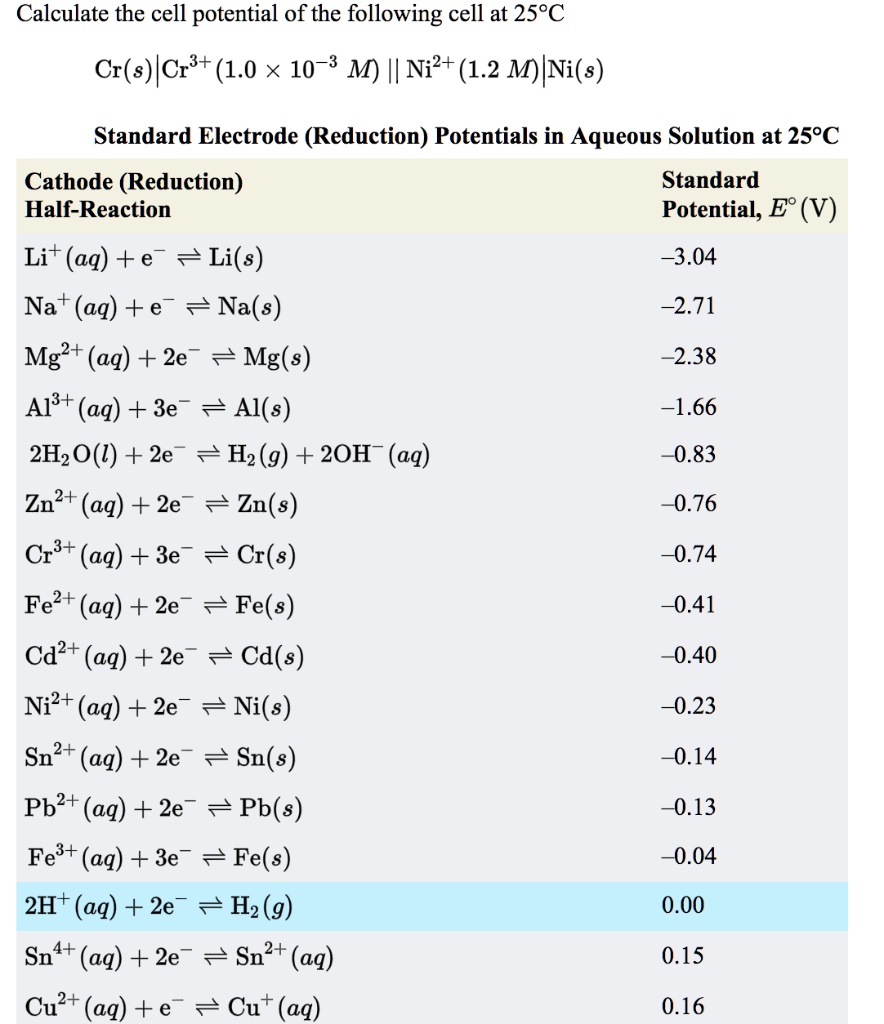
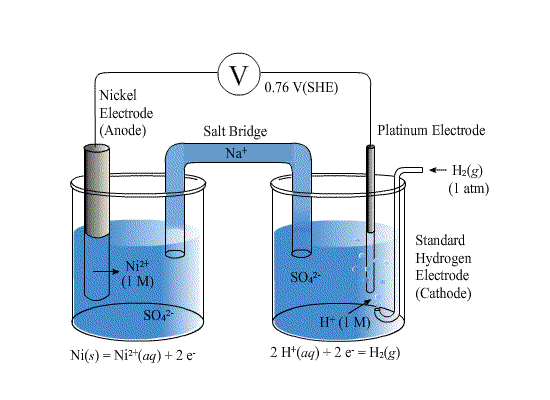
SOLVED: Calculate the cell potential of the following cell at 258C Cr(s)ICr8+(1.0 10-: M) || Ni2+ (1.2 M) Ni(s) Standard Electrode (Reduction) Potentials in Aqueous Solution at 259€ Cathode (Reduction) Standard Half-Reaction

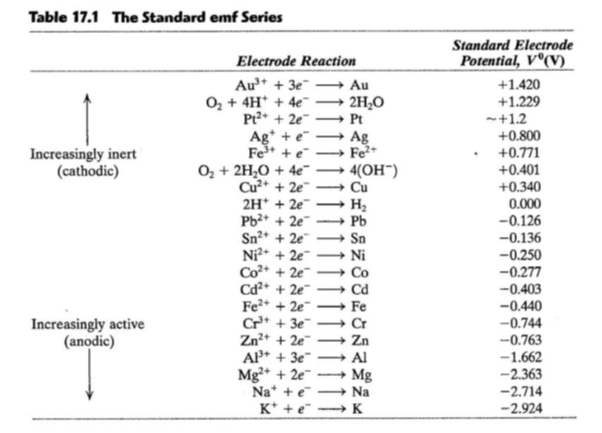
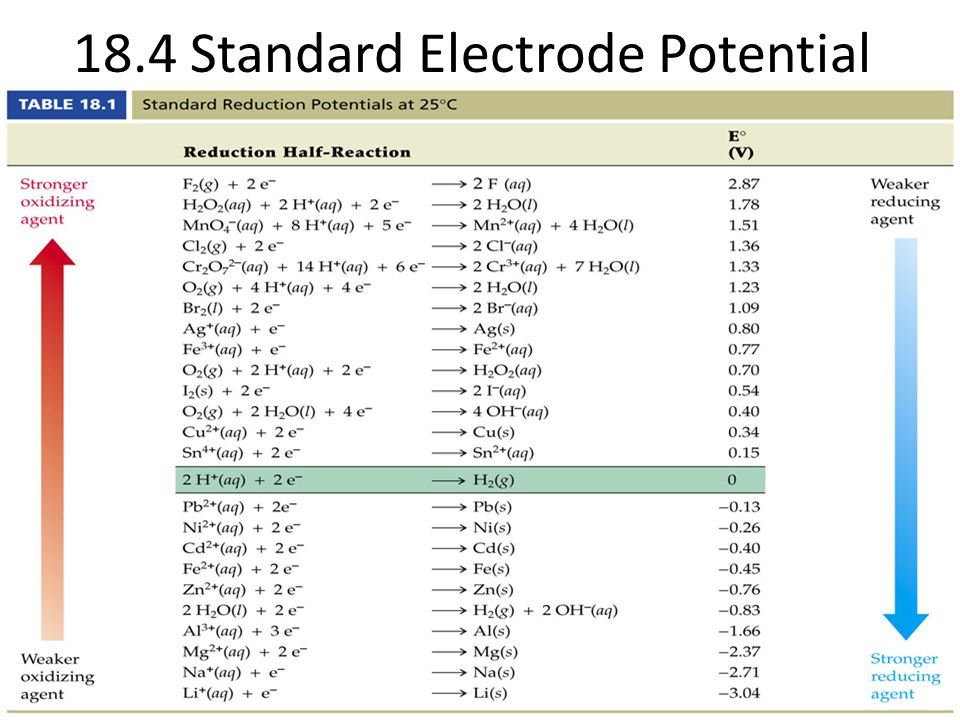
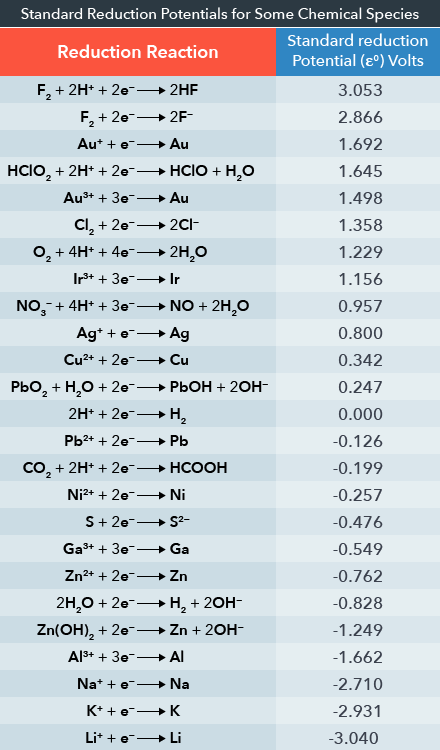
Chemistry - ELECTROCHEMICAL SERIES AND ITS APPLICATION:- A list of elements arranged in order on the basis of their standard reduction potential or oxidation potential is called electrochemical series. EXPLAINATION:- Different elements

Calculate the standard electrode potential of the Ni^(2+)//Ni electrode , if the cell potential ... - YouTube

Calculate the standard electrode potential of Ni2+/Ni electrode if |Class 12 CHEMISTRY | Doubtnut - YouTube

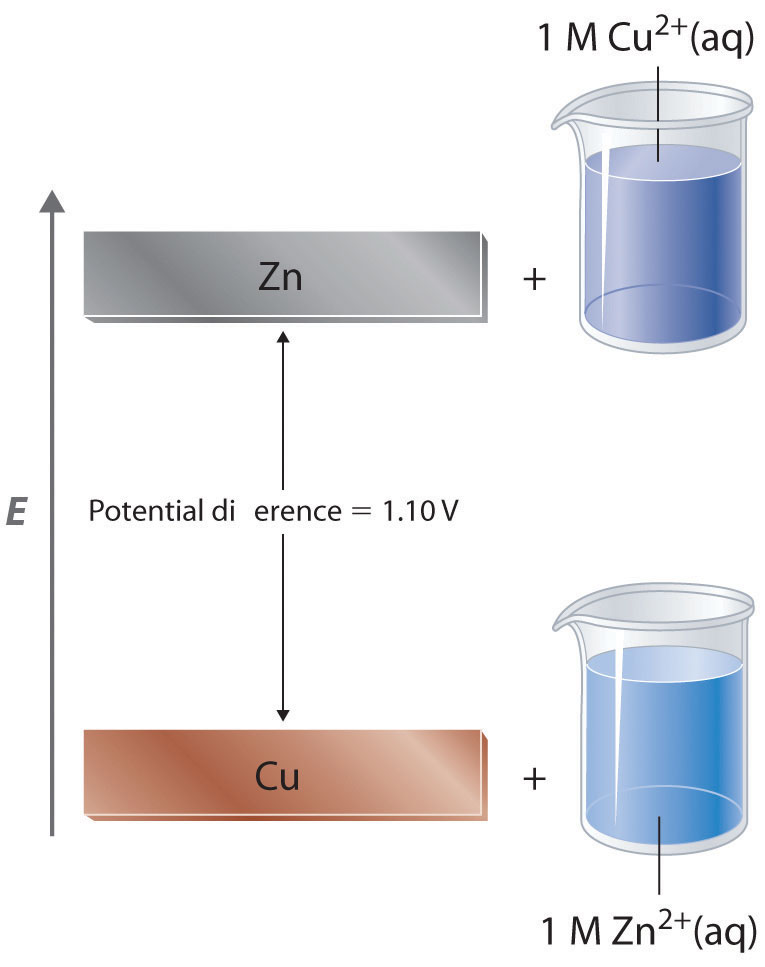
The EMF of the cell Ni `|Ni|Ni^(2+) ||Cu^(2+) |Cu(s)` is `0.59 ` volt. The standard reduction - YouTube
![Calculate the standard electrode potential of Ni2+/Ni electrode if emf of the cell Ni |Ni2+(0.01 M)||Cu2+(0.1 M)| Cu(s) is 0.59 V at 25°C [Given: Ecu2+Icu = +0.34 v] Calculate the standard electrode potential of Ni2+/Ni electrode if emf of the cell Ni |Ni2+(0.01 M)||Cu2+(0.1 M)| Cu(s) is 0.59 V at 25°C [Given: Ecu2+Icu = +0.34 v]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/644872050_web.png)
Calculate the standard electrode potential of Ni2+/Ni electrode if emf of the cell Ni |Ni2+(0.01 M)||Cu2+(0.1 M)| Cu(s) is 0.59 V at 25°C [Given: Ecu2+Icu = +0.34 v]
The standard oxidation potential of Ni|Ni2+ electrode = 0.236 V. If this is combined with a hydrogen electrode in acid solution, at what pH of the solution will the measured emf be

Calculate the standard electrode potential of Ni2+/Ni electrode if emf of the cell Ni(s)/Ni2+/Ni2+(0 01 m)II Cu2+(0 1M)I Cu(s) is - Chemistry - - 4081769 | Meritnation.com

The standard electrode potential of two half cells are given below - Ni^+2 + 2e^ - ⟶ Ni; E^0 = - 0.25V Zn^+2 + 2e^ - ⟶ Zn; E^0 = - 0.77V