Explain the Ni(CO)4 is tetrahedral but [Ni(CN)4]^2– is square planar. - Sarthaks eConnect | Largest Online Education Community
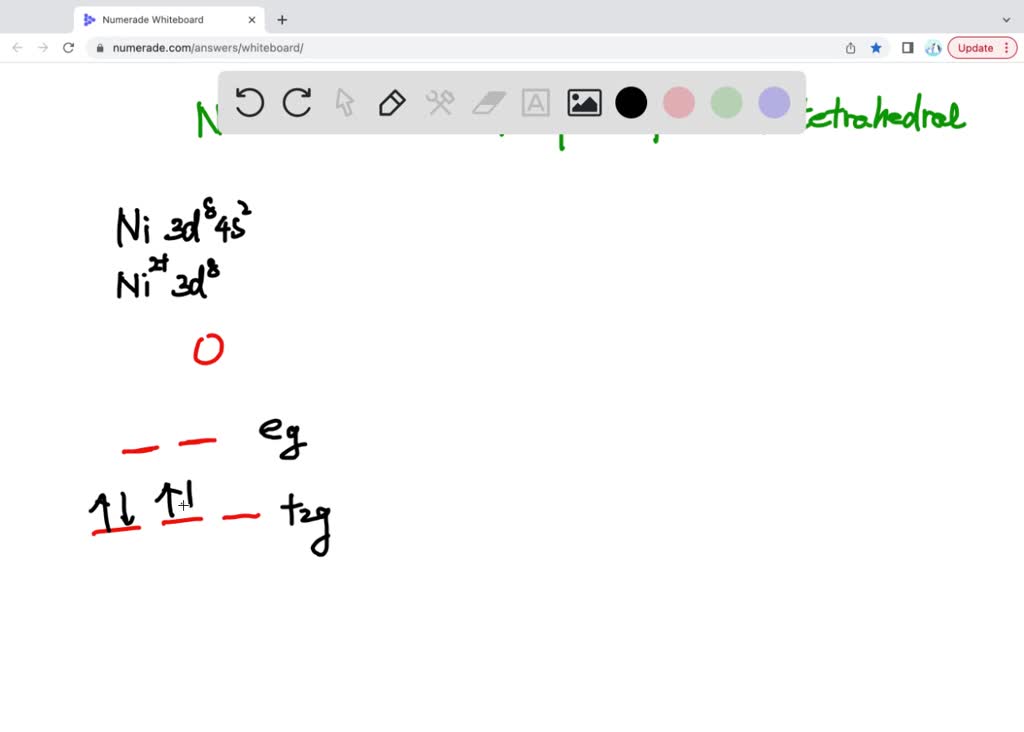
SOLVED: Nickel (II) forms octahedral, square planar and tetrahedral complexes with very different colours typically onserved. Draw the crystal field splitting diagrams showing:a) the electronic state of each of theseb) the electron

Square Planar vs Tetrahedral Coordination in Diamagnetic Complexes of Nickel(II) Containing Two Bidentate π-Radical Monoanions | Inorganic Chemistry
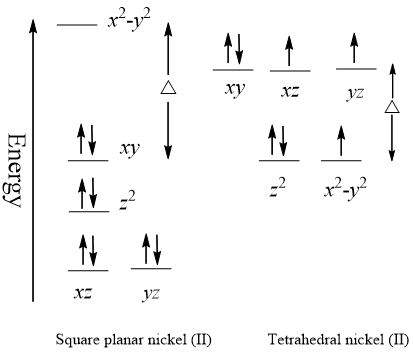
Solved: Chapter 20 Problem 120P Solution | Student Solutions Manual For General Chemistry 2nd Edition | Chegg.com

Impact of tetrahedral and square planar geometry of Ni(II) complexes with (pseudo)halide ligands to magnetic properties - ScienceDirect

Square Planar vs Tetrahedral Coordination in Diamagnetic Complexes of Nickel(II) Containing Two Bidentate π-Radical Monoanions | Inorganic Chemistry

Suggested structure of the square planar Ni(II) complex of the ligand,... | Download Scientific Diagram
![The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com](https://homework.study.com/cimages/multimages/16/cms53022462554618608855.jpg)
The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com
Geometrical shapes of complexes formed by the reaction of ${\\text{N}}{{\\text{i}}^{{\\text{ + 2}}}}$ with ${\\text{C}}{{\\text{l}}^{\\text{ - }}}{\\text{,C}}{{\\text{N}}^{\\text{ - }}}$and ${{\\text{H}}_{\\text{2}}}{\\text{O}}$, respectively are:(A ...
![The complex `[NiCl_(4)]^(2-)` has tetrahedral geometry while `[Ni(CN)_(4)]^(2-)` has square planar - YouTube The complex `[NiCl_(4)]^(2-)` has tetrahedral geometry while `[Ni(CN)_(4)]^(2-)` has square planar - YouTube](https://i.ytimg.com/vi/JCDuVCjb-Uk/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLABPYxM2ekkgRxLQOhhqKsuv5imwQ)
The complex `[NiCl_(4)]^(2-)` has tetrahedral geometry while `[Ni(CN)_(4)]^(2-)` has square planar - YouTube
![NI(Cl)2(P(CH3)3)2] is a paramagnetic complex of Ni(II), Analgous Pd(II) complex is diamagnetic. How many geometrical isomers will be possible for Ni(II) and Pd(II) complexes? Also explain their magnetic behaviour. NI(Cl)2(P(CH3)3)2] is a paramagnetic complex of Ni(II), Analgous Pd(II) complex is diamagnetic. How many geometrical isomers will be possible for Ni(II) and Pd(II) complexes? Also explain their magnetic behaviour.](https://haygot.s3.amazonaws.com/questions/1292483_256043_ans_a5318a12ca584fbfa4350ce3909aa29c.jpg)
NI(Cl)2(P(CH3)3)2] is a paramagnetic complex of Ni(II), Analgous Pd(II) complex is diamagnetic. How many geometrical isomers will be possible for Ni(II) and Pd(II) complexes? Also explain their magnetic behaviour.

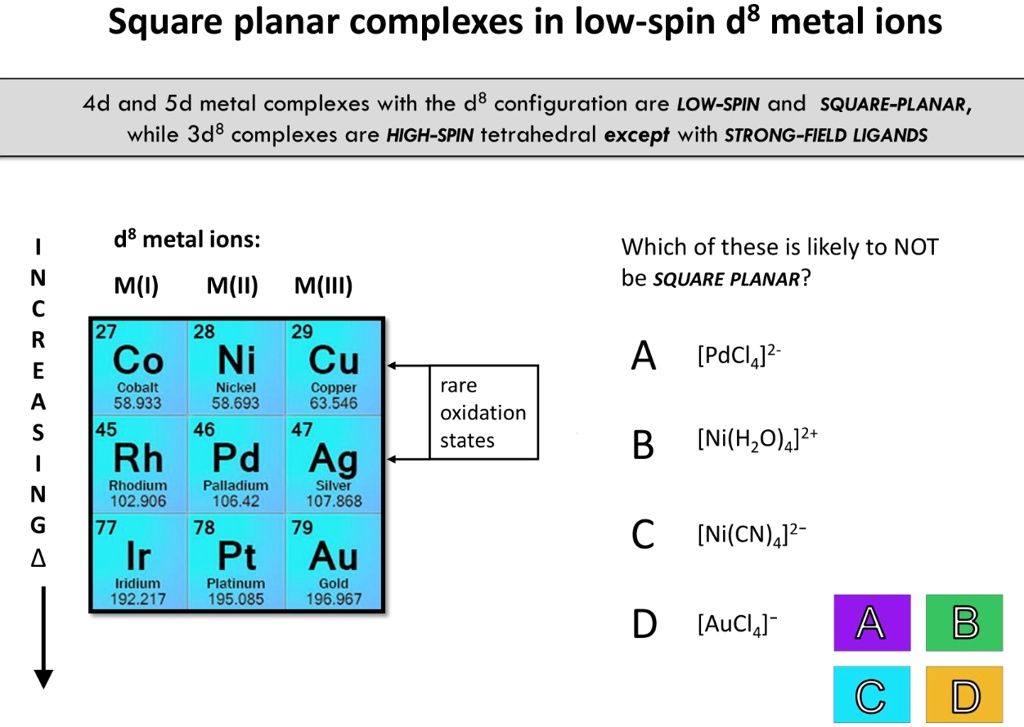
Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-2.png)

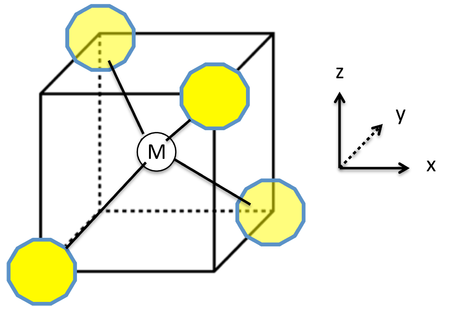

![Ni(CO)4, [Ni(CN)4]2-, [NiCl4]2-Structure-Hybridization-VBT-IIT JEE NEET SAT NCERT CBSE - YouTube Ni(CO)4, [Ni(CN)4]2-, [NiCl4]2-Structure-Hybridization-VBT-IIT JEE NEET SAT NCERT CBSE - YouTube](https://i.ytimg.com/vi/r_C4yyTUSjM/maxresdefault.jpg)
![The geometry and magnetic behaviour of the complex [Ni(CO)4] are (a)s The geometry and magnetic behaviour of the complex [Ni(CO)4] are (a)s](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/643994163_web.png)
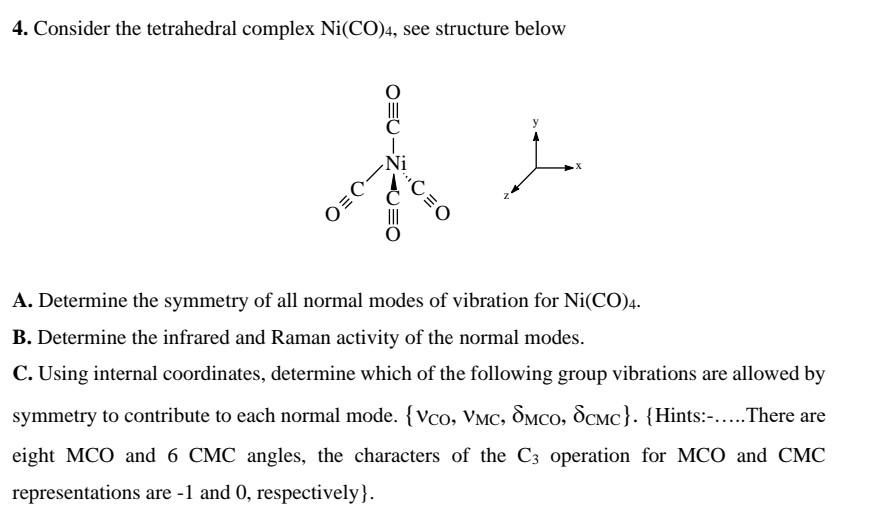
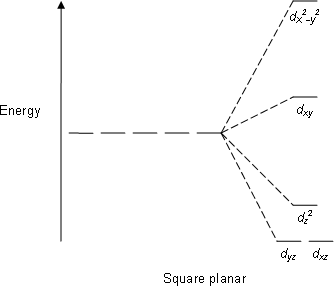
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-1.png)
![coordination compounds - Why is Ni[(PPh₃)₂Cl₂] tetrahedral? - Chemistry Stack Exchange coordination compounds - Why is Ni[(PPh₃)₂Cl₂] tetrahedral? - Chemistry Stack Exchange](https://i.stack.imgur.com/AD72m.png)
