Quinone 1 e– and 2 e–/2 H+ Reduction Potentials: Identification and Analysis of Deviations from Systematic Scaling Relationships | Journal of the American Chemical Society
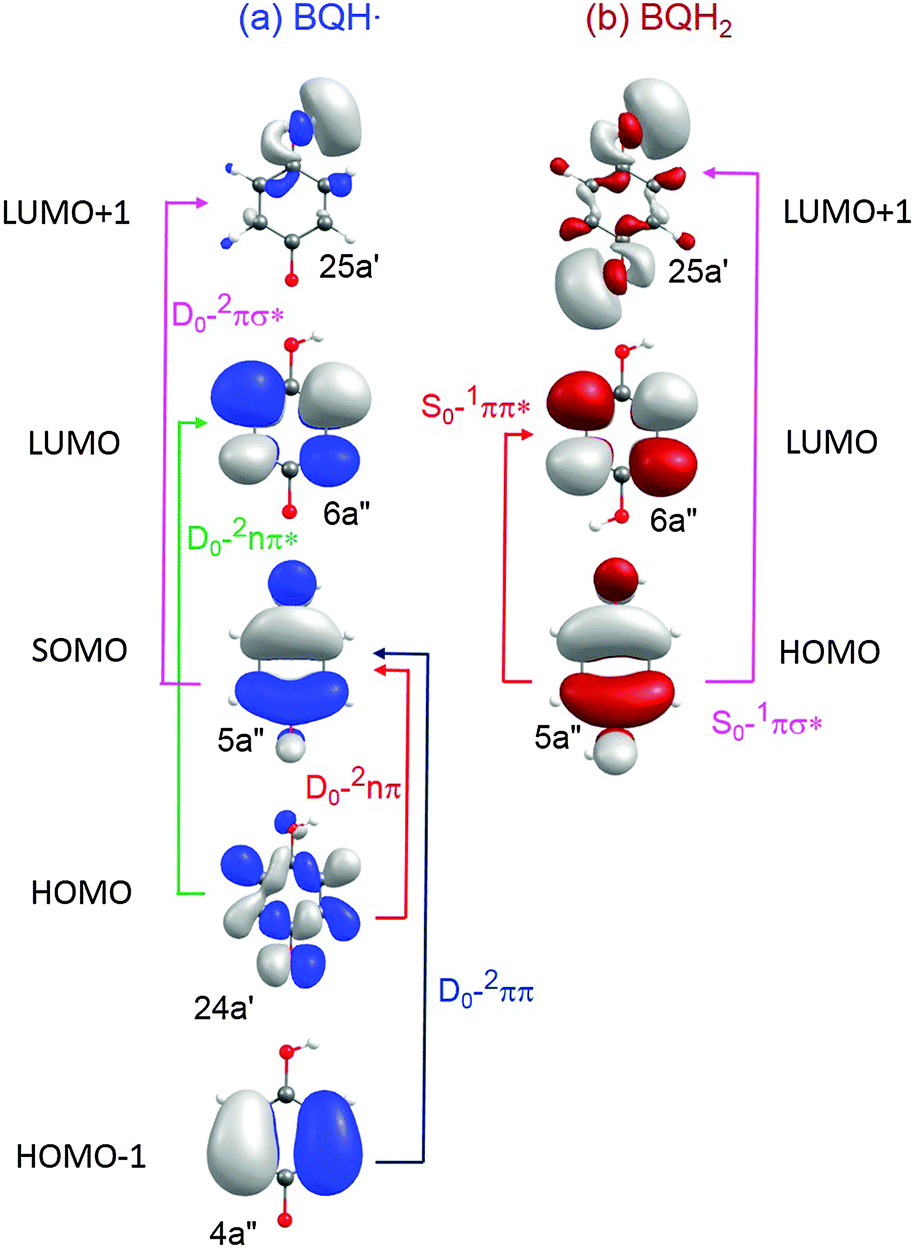
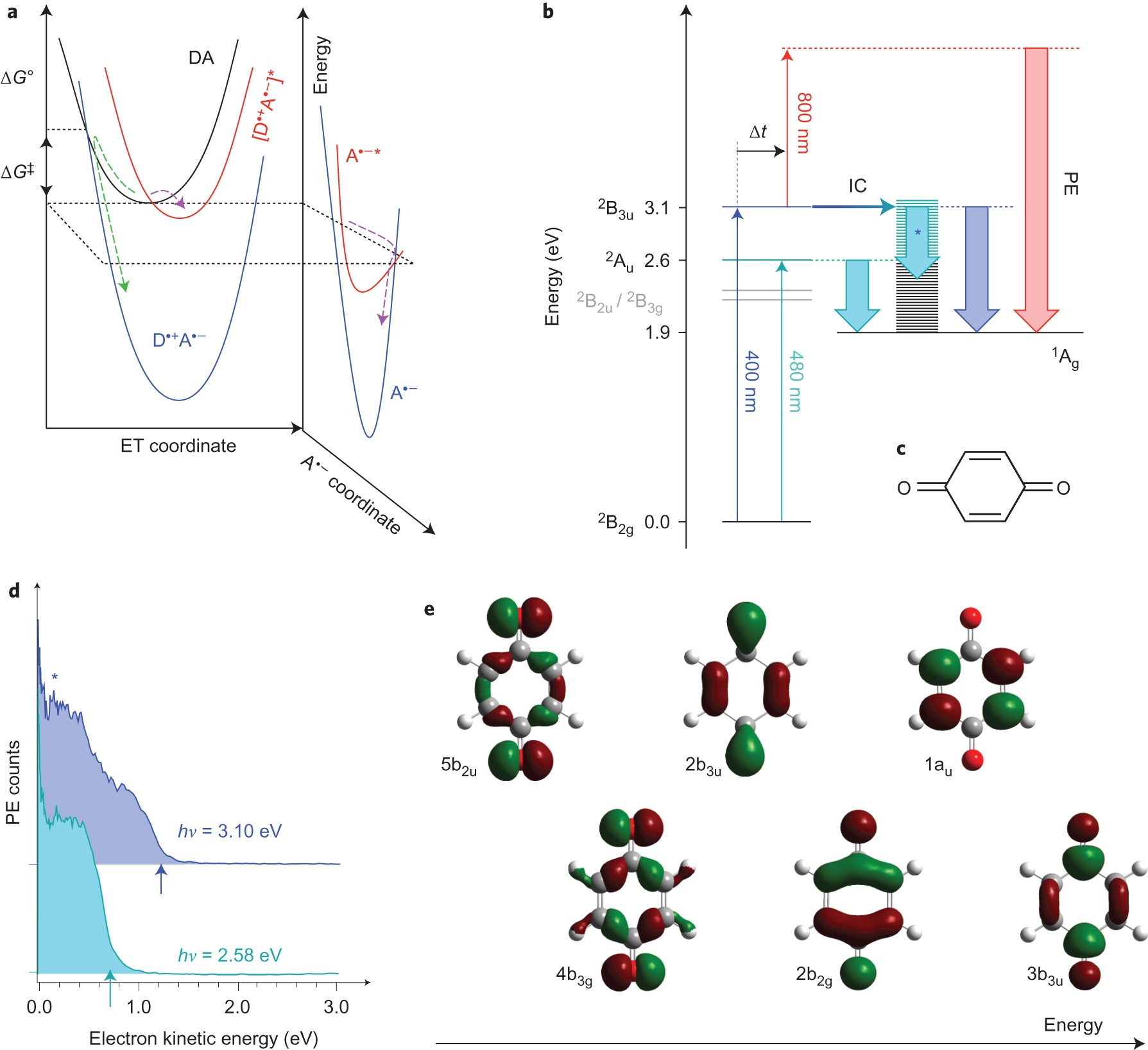
Photoinduced water splitting via benzoquinone and semiquinone sensitisation - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C5CP03831F

Quinone 1 e– and 2 e–/2 H+ Reduction Potentials: Identification and Analysis of Deviations from Systematic Scaling Relationships | Journal of the American Chemical Society
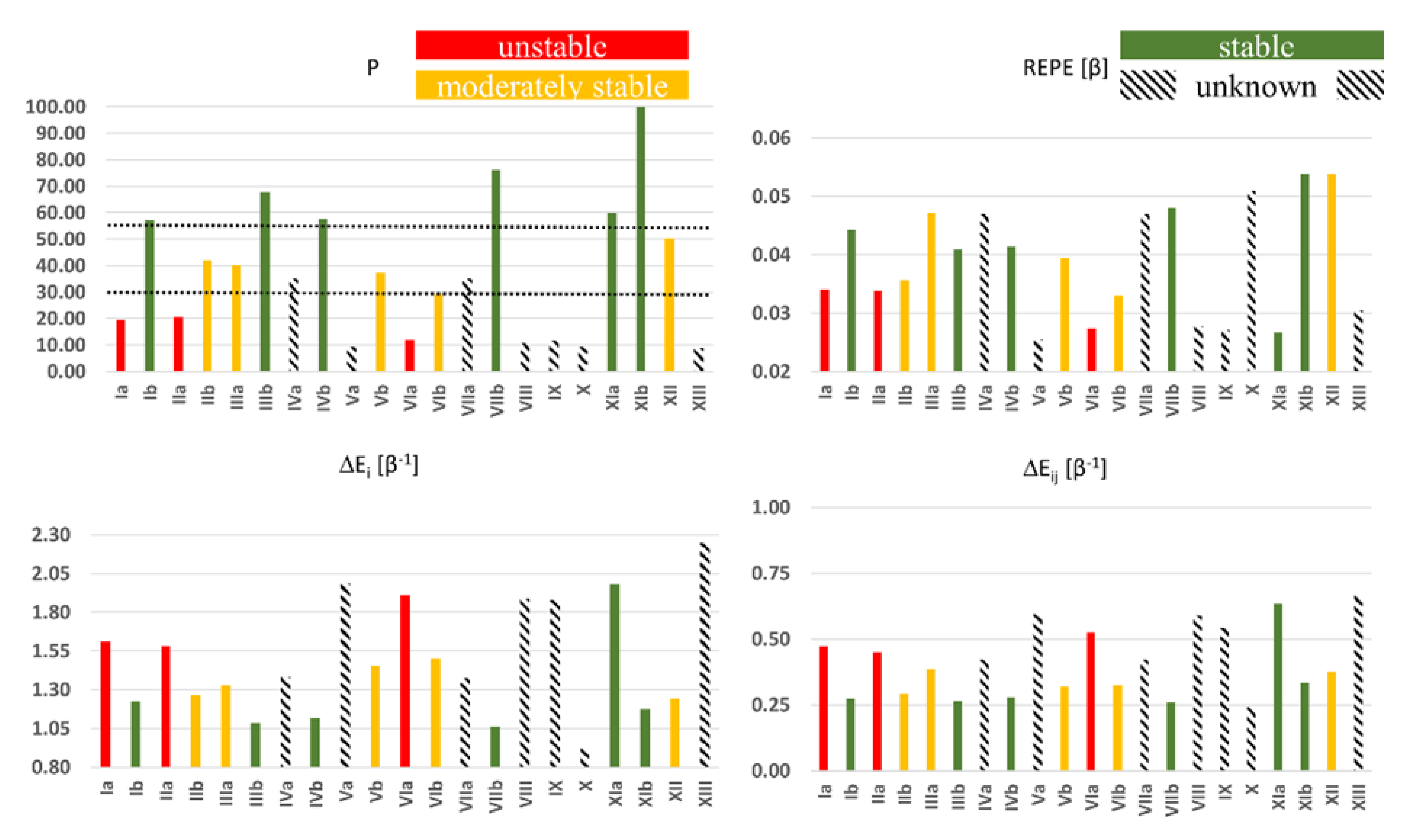
Symmetry | Free Full-Text | The Interplay between Diradical Character and Stability in Organic Molecules

Polymers containing in‐chain quinone moieties: synthesis and properties - Hodge - 2009 - Polymer International - Wiley Online Library

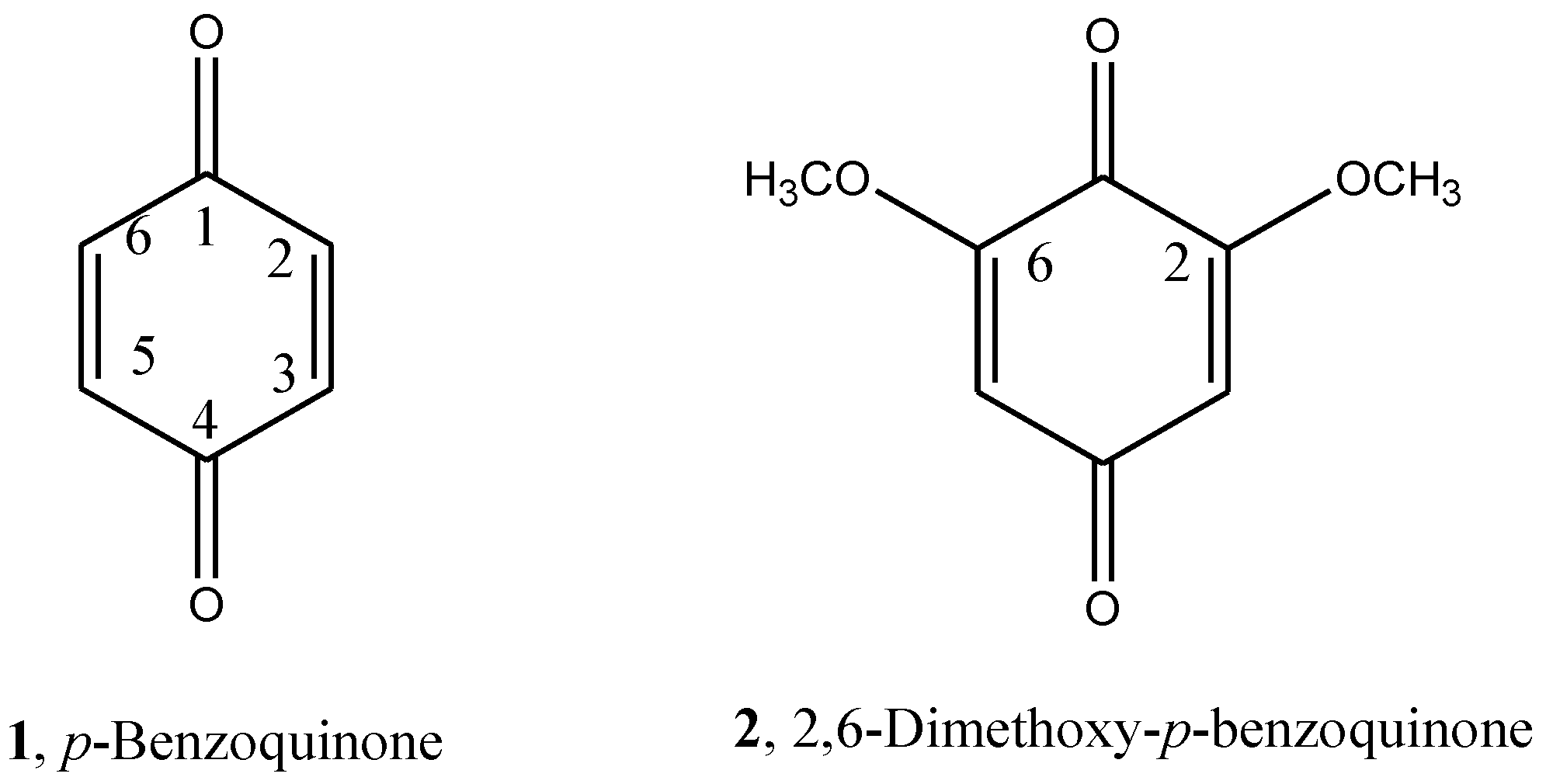
Substituent Pattern Effects on the Redox Potentials of Quinone‐Based Active Materials for Aqueous Redox Flow Batteries - Schwan - 2020 - ChemSusChem - Wiley Online Library

PDF) Remote Position Substituents as Modulators of Conformational and Reactive Properties of Quinones. Relevance of the π/π Intramolecular Interaction | GABRIEL PIZARRO CUEVAS - Academia.edu
Pyrrole-bridged quinones: π-electronic systems that modulate electronic structures by tautomerism and deprotonation - Chemical Communications (RSC Publishing)

Rh(III)‐Catalyzed Redox‐Neutral Cascade Annulation of Benzamides with p‐ Quinone Methides - Kanchupalli - 2020 - European Journal of Organic Chemistry - Wiley Online Library