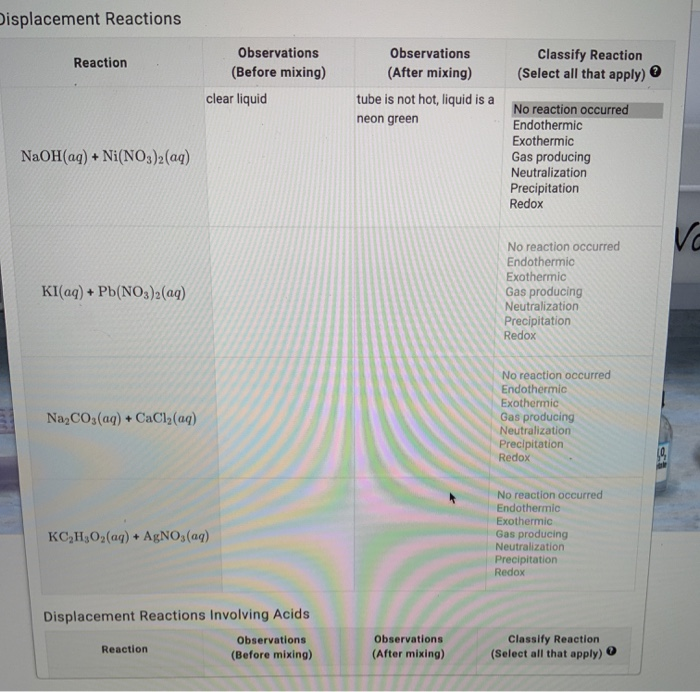
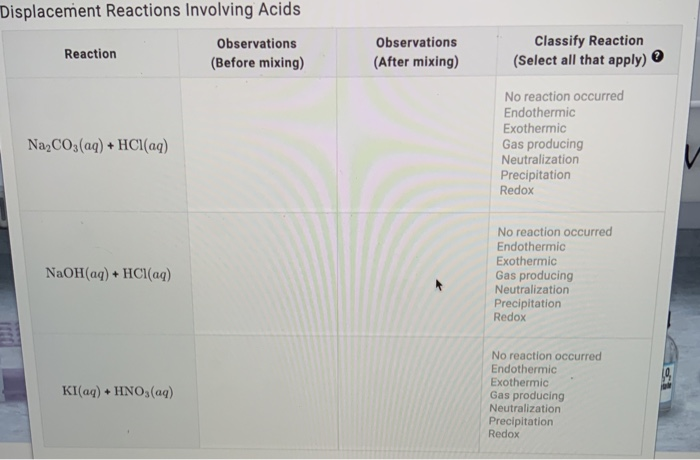
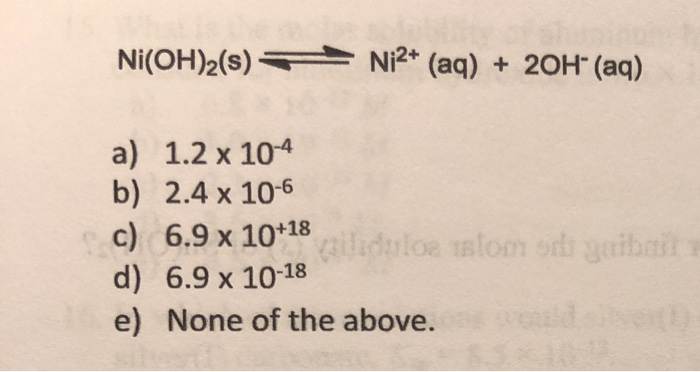SOLVED: The molar solubility of nickel(II) hydroxide (Ni(OH)2) is 2.8 x 10-6 mol/L in pure water at 25°C. What is the molar solubility of nickel(II) hydroxide in 0.050 M NaOH at 25°C? (

A) FTIR spectra and (B) TG curves of (a) Ni 3 Si 2 O 5 (OH) 4 , (b) Ni... | Download Scientific Diagram

Calculate the molar solubility of Ni(OH)2 in 0.10M NaOH. The ionic product of Ni(OH)2 is..... - YouTube
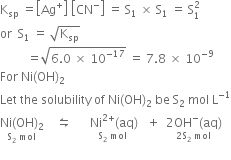
The values of Ksp of two sparingly soluble salts Ni(OH)2 and AgCN are 2·0 × 10–15 and 6 × 10–17 respectively. Which salt is more soluble ? Explain from Chemistry Equilibrium Class 11 CBSE

SOLVED: 22)For a certain reaction AHO= -75.4 kJ and Aso=-130.0 J/K: Is the reaction endothermic or exothermic? Does the reaction lead to an increase or decrease in the disorder ofthe system? Calculate
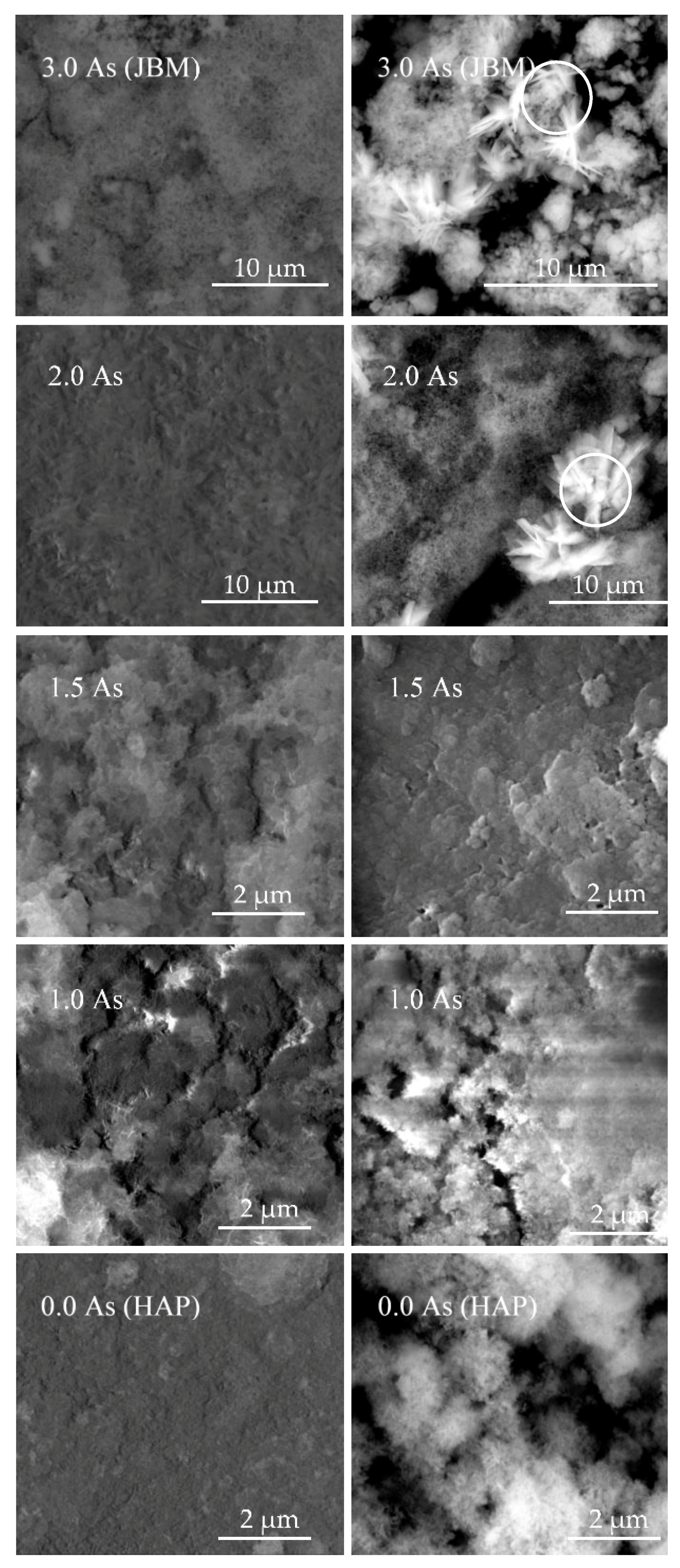
Minerals | Free Full-Text | Transition from Endothermic to Exothermic Dissolution of Hydroxyapatite Ca5(PO4)3OH–Johnbaumite Ca5(AsO4)3OH Solid Solution Series at Temperatures Ranging from 5 to 65 °C

Solubility of NiO (cr) at infinite dilution versus pH° m at temperature... | Download Scientific Diagram